First-Feeding Cod Larvae Live for Microzooplankton
First-Feeding Cod Larvae Live for Microzooplankton
Scott M. Gallager, Jeff Van Keuren
and Phillip Alatalo
Historically, it has been thought that planktotrophic
teleost larvae begin and continue to feed following
yolk-sac absorption, a vitally critical period requiring prey to be
available of optimal size, chemical composition and concentration.
Copepod nauplii are usually considered the primary 'first food' for
young cod larvae because they are the first hard-bodied organisms
observed in the larval guts. Using a novel technique for
quantifying ingestion of protozoa by larval fish (Fig. 1),
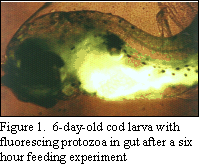 we have
shown that cod larvae Gadus morhua feed extensively on
soft-bodied protozoans well before the yolk-sac is absorbed. This
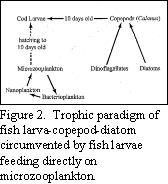
circumvents the classical food chain paradigm leading to a direct
link between members of the microbial loop and higher trophic levels
(Fig. 2).
we have
shown that cod larvae Gadus morhua feed extensively on
soft-bodied protozoans well before the yolk-sac is absorbed. This
circumvents the classical food chain paradigm leading to a direct
link between members of the microbial loop and higher trophic levels
(Fig. 2).
 Our results show that newly-hatched cod larvae require
soft-bodied microzooplankton in the size range of 40 to 80 m at a
nominal concentration of about 2 cells ml-1 in order to
maximize survival through yolk-sac absorption (~10-15 days
post-hatch). Furthermore, the down-welling light field, mixing
intensity (turbulence), and the motion of the prey organism can have
a dramatic effect on successful foraging by young cod larvae.
Optimal light wavelength and light intensity for foraging in young
cod larvae appears to center around 520 nm and 10 to 80 W
m-2s-1, respectively (Fig. 3).
Our results show that newly-hatched cod larvae require
soft-bodied microzooplankton in the size range of 40 to 80 m at a
nominal concentration of about 2 cells ml-1 in order to
maximize survival through yolk-sac absorption (~10-15 days
post-hatch). Furthermore, the down-welling light field, mixing
intensity (turbulence), and the motion of the prey organism can have
a dramatic effect on successful foraging by young cod larvae.
Optimal light wavelength and light intensity for foraging in young
cod larvae appears to center around 520 nm and 10 to 80 W
m-2s-1, respectively (Fig. 3).
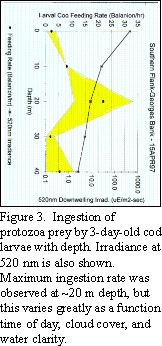 This
combination of light quality and intensity may occur at depths of 30
m to near surface depending on time of day, cloud cover and the
water's attenuation coefficient. As the larvae move vertically in
the water column following optimal light conditions, they are
exposed to a variety of different prey species and physical mixing
conditions.
This
combination of light quality and intensity may occur at depths of 30
m to near surface depending on time of day, cloud cover and the
water's attenuation coefficient. As the larvae move vertically in
the water column following optimal light conditions, they are
exposed to a variety of different prey species and physical mixing
conditions.
Under calm conditions, some protozoans can form dense layers or
patches near the pycnocline where larval fish are exposed to an
enhanced feeding environment (Fig. 4). As turbulence increases,
through either wind mixing from the surface or by passage of an
internal wave, the layers of prey are dispersed thereby reducing
prey 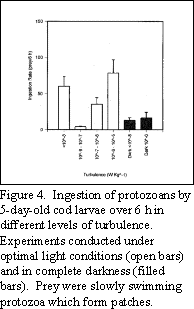 concentrations available to the larvae and consequently
their feeding rates. If turbulence increases even further,
ingestion rates increase again as the effective prey concentration
increases as a function of the increased contact rate between larva
and prey as provided by small-scale eddies. Although not shown in
Fig. 4, very high turbulence, such as would be found near the
surface during a storm (i.e., >10-5 W
Kg-1), results in complete disruption of the foraging
process leading to larval starvation.
concentrations available to the larvae and consequently
their feeding rates. If turbulence increases even further,
ingestion rates increase again as the effective prey concentration
increases as a function of the increased contact rate between larva
and prey as provided by small-scale eddies. Although not shown in
Fig. 4, very high turbulence, such as would be found near the
surface during a storm (i.e., >10-5 W
Kg-1), results in complete disruption of the foraging
process leading to larval starvation.
While some protozoan prey swim slowly, predictably, and
tend to form patches, others swim quickly and do not aggregate in
response to density or light gradients. When larvae are exposed to
such prey, an increase in turbulence only tends to decrease feeding
rates (Fig. 5).
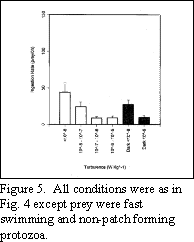 Presumably, this is due to the combination of the
prey's fast swim speed and the elevated turbulent velocity which
leads to disruption of the foraging process. Note that in both
Figures 4 and 5, ingestion by larval cod in the dark was virtually
independent of turbulence suggesting that vision is crucial for
detecting prey under elevated contact rates.
Presumably, this is due to the combination of the
prey's fast swim speed and the elevated turbulent velocity which
leads to disruption of the foraging process. Note that in both
Figures 4 and 5, ingestion by larval cod in the dark was virtually
independent of turbulence suggesting that vision is crucial for
detecting prey under elevated contact rates.
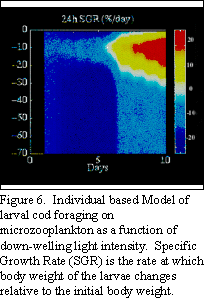 In conjunction with John Quinlan and Cisco Werner at the
University of North Carolina, we have been modeling the effects of
the light field and turbulence on foraging in early larval cod in
relation to the microzooplankton prey field. During the months of
January through March on the Northeast Peak of Georges Bank, the
abundance of microzooplankton prey typically exceeds minimum levels
for good growth in early cod larvae. Model results show, however,
that foraging depends strongly on the ambient light field which may
be optimal for only a few short hours each day under the best of
conditions, and perhaps not at all under cloudy and stormy
conditions (Fig. 6). These and further results are being
incorporated into a 3-D advection-diffusion model to determine
growth and mortality rates of young cod as they are transported
around the bank.
In conjunction with John Quinlan and Cisco Werner at the
University of North Carolina, we have been modeling the effects of
the light field and turbulence on foraging in early larval cod in
relation to the microzooplankton prey field. During the months of
January through March on the Northeast Peak of Georges Bank, the
abundance of microzooplankton prey typically exceeds minimum levels
for good growth in early cod larvae. Model results show, however,
that foraging depends strongly on the ambient light field which may
be optimal for only a few short hours each day under the best of
conditions, and perhaps not at all under cloudy and stormy
conditions (Fig. 6). These and further results are being
incorporated into a 3-D advection-diffusion model to determine
growth and mortality rates of young cod as they are transported
around the bank.
 we have
shown that cod larvae Gadus morhua feed extensively on
soft-bodied protozoans well before the yolk-sac is absorbed. This
circumvents the classical food chain paradigm leading to a direct
link between members of the microbial loop and higher trophic levels
(Fig. 2).
we have
shown that cod larvae Gadus morhua feed extensively on
soft-bodied protozoans well before the yolk-sac is absorbed. This
circumvents the classical food chain paradigm leading to a direct
link between members of the microbial loop and higher trophic levels
(Fig. 2).
 Our results show that newly-hatched cod larvae require
soft-bodied microzooplankton in the size range of 40 to 80 m at a
nominal concentration of about 2 cells ml-1 in order to
maximize survival through yolk-sac absorption (~10-15 days
post-hatch). Furthermore, the down-welling light field, mixing
intensity (turbulence), and the motion of the prey organism can have
a dramatic effect on successful foraging by young cod larvae.
Optimal light wavelength and light intensity for foraging in young
cod larvae appears to center around 520 nm and 10 to 80 W
m-2s-1, respectively (Fig. 3).
Our results show that newly-hatched cod larvae require
soft-bodied microzooplankton in the size range of 40 to 80 m at a
nominal concentration of about 2 cells ml-1 in order to
maximize survival through yolk-sac absorption (~10-15 days
post-hatch). Furthermore, the down-welling light field, mixing
intensity (turbulence), and the motion of the prey organism can have
a dramatic effect on successful foraging by young cod larvae.
Optimal light wavelength and light intensity for foraging in young
cod larvae appears to center around 520 nm and 10 to 80 W
m-2s-1, respectively (Fig. 3).
 This
combination of light quality and intensity may occur at depths of 30
m to near surface depending on time of day, cloud cover and the
water's attenuation coefficient. As the larvae move vertically in
the water column following optimal light conditions, they are
exposed to a variety of different prey species and physical mixing
conditions.
This
combination of light quality and intensity may occur at depths of 30
m to near surface depending on time of day, cloud cover and the
water's attenuation coefficient. As the larvae move vertically in
the water column following optimal light conditions, they are
exposed to a variety of different prey species and physical mixing
conditions.  concentrations available to the larvae and consequently
their feeding rates. If turbulence increases even further,
ingestion rates increase again as the effective prey concentration
increases as a function of the increased contact rate between larva
and prey as provided by small-scale eddies. Although not shown in
Fig. 4, very high turbulence, such as would be found near the
surface during a storm (i.e., >10-5 W
Kg-1), results in complete disruption of the foraging
process leading to larval starvation.
concentrations available to the larvae and consequently
their feeding rates. If turbulence increases even further,
ingestion rates increase again as the effective prey concentration
increases as a function of the increased contact rate between larva
and prey as provided by small-scale eddies. Although not shown in
Fig. 4, very high turbulence, such as would be found near the
surface during a storm (i.e., >10-5 W
Kg-1), results in complete disruption of the foraging
process leading to larval starvation.  Presumably, this is due to the combination of the
prey's fast swim speed and the elevated turbulent velocity which
leads to disruption of the foraging process. Note that in both
Figures 4 and 5, ingestion by larval cod in the dark was virtually
independent of turbulence suggesting that vision is crucial for
detecting prey under elevated contact rates.
Presumably, this is due to the combination of the
prey's fast swim speed and the elevated turbulent velocity which
leads to disruption of the foraging process. Note that in both
Figures 4 and 5, ingestion by larval cod in the dark was virtually
independent of turbulence suggesting that vision is crucial for
detecting prey under elevated contact rates.  In conjunction with John Quinlan and Cisco Werner at the
University of North Carolina, we have been modeling the effects of
the light field and turbulence on foraging in early larval cod in
relation to the microzooplankton prey field. During the months of
January through March on the Northeast Peak of Georges Bank, the
abundance of microzooplankton prey typically exceeds minimum levels
for good growth in early cod larvae. Model results show, however,
that foraging depends strongly on the ambient light field which may
be optimal for only a few short hours each day under the best of
conditions, and perhaps not at all under cloudy and stormy
conditions (Fig. 6). These and further results are being
incorporated into a 3-D advection-diffusion model to determine
growth and mortality rates of young cod as they are transported
around the bank.
In conjunction with John Quinlan and Cisco Werner at the
University of North Carolina, we have been modeling the effects of
the light field and turbulence on foraging in early larval cod in
relation to the microzooplankton prey field. During the months of
January through March on the Northeast Peak of Georges Bank, the
abundance of microzooplankton prey typically exceeds minimum levels
for good growth in early cod larvae. Model results show, however,
that foraging depends strongly on the ambient light field which may
be optimal for only a few short hours each day under the best of
conditions, and perhaps not at all under cloudy and stormy
conditions (Fig. 6). These and further results are being
incorporated into a 3-D advection-diffusion model to determine
growth and mortality rates of young cod as they are transported
around the bank.